Abstract
Background: Refractory and relapsed (r/r) anaplastic lymphoma kinase (ALK)-positive anaplastic large cell Lymphoma(ALK+ALCL) have a poor prognosis. Studies had confirmed vinblastine (VBL) may be an effective treatment for relapsed ALCL and ALK inhibitors may have high efficacy and tolerability in patients carrying ALK fusions. Therefore, we put forward a hypothesis that for those r/r ALK+ALCL patients with poor chemotherapy tolerance and effect, combining ALK inhibitors with vinblastine may have a sustained anti-tumor effect and good tolerance.
Aims: To assess the efficacy and safety of ALK inhibitors combined with VBL in pediatric relapsed/refractory (r/r) ALK+ ALCL.
Methods: Patients aged between 0 and 18 years with r/r ALK+ ALCL were included in this trial. ALK inhibitors are given orally with a converted dosage according to body surface area
and VBL was given with injection once a week at 4-6 mg/(m2.week). And no intensive chemotherapy and hematopoietic stem cell transplantation will be in our study.We may decrease the dosage of VBL or delete VBL if patients suffer severe bone marrow suppression, repeated infections, and peripheral neuritis and other VBL-related toxicities. Available ALK inhibitors included crizotinib(CZ), alectinib(AL) and ceritinib(CER). Patients without CNS involvement took CZ, and patients with CNS involvement took AL or CER. A cycle of therapy is 28 days. Evaluation for responses toxicities were monitored and recorded.
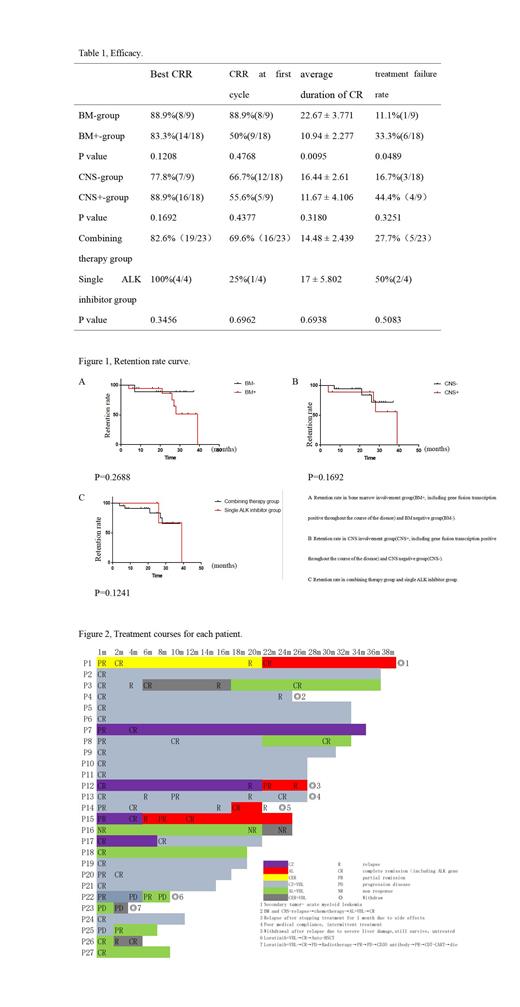
Results:27 patients were enrolled in this trial between April 2018 and April 2021. The median age was 8.3 years old(ranging from 0.75 to 16), male/female ratio is19:8. By St.jude's staging, 20 cases were in stage III (74.1%), 7 cases in stage IV (25.9%) including 3 cases (11.1%) with CNS involvement at initial diagnosis. Before being enrolled in our study, 6 patient (22.2%) failed to achieve CR with prior 4-10 chemotherapy regimens, 21 patients (77.8%) relapsed after an average of 5-16 courses of standard pulse chemotherapy regimens. After 1st cycle of treatments, 62.9%(17/26) achieved comlpele remission(CR) without measurable tumors and ALK gene transcription, no progressed cases. Additional 6 case got CR after 2-10 month(23.1%). In general, 7 patients withdraw due to death, progress, ineffectiveness, economics, adverse reactions. The treatment retention rate was ,7.1%(20/7),objective response rate (ORR) was 85.2%(23/27), best complete remission rate(CRR) was 85.2%(23/27), disease control rate(DCR) was 92.5%(25/27), average duration of CR was 14.85 months(0-36months).
Toxicities included nausea and vomiting, stomachache, diarrhea(81.5%,22/27, one or more above toxicities), neutropenia (27/27), pneumonia(7.4%, 2/27), sepsis(7.4%, 2/27), creatine kinase-MB elevation (81.5%,22/27,all are in crizotinib group including 2 abnormal ECG cases), liver enzyme elevation (63.%,17//27), intestinal obstruction(7.4%, 2/26). 2 cases relapsed because of the treatment discontinuation due to severe elevated liver enzymes.
Summary/Conclusion: ALK inhibitors combined with VBL may rapidly induce the tumor remission of r/r ALK+ALCL and the tolerance is good. Combination therapy may induce a more lasting remission in for patients without BM involvement. Patients with BM involvement are more likely to fail treatment. Sequential use of ALK kinase inhibitors may be an efficient strategy to counter drug resistance.More data and longer follow-up time are needed to support our study.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal